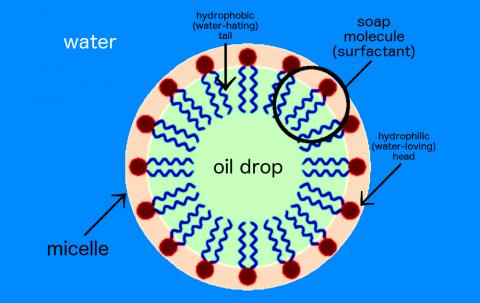
Oil and water do not mix, but if we add soap to a solution of oil and water, the soap can break down the oil. How is this possible?
This illustration and its description, from America's Brookhaven National Lab, depicts and explains how the process begins:
Soap is a surfactant, or surface-active agent. One end of the soap molecule is hydrophilic (water-loving) and thus binds to water; the other end is hydrophobic (water-hating) and binds to oil molecules. In water, soap molecules form a ring around the drop of oil. This structure is called a micelle.
Click on the illustration to enlarge it.